New perspectives
The changing shape of abutments and implants: future evolution
Implant surface modifications
There are four methods for modifying surface properties on implants: topographical, chemical, physical and mechanical. The first two have been the subject of particular interest in recent years.
Topographic modifications
The market is now dominated by moderately rough isotropic surfaces. But now a question arises: could they also be harmful? The speaker posited two questions:
- Is it true that the rougher the surface, the higher the risk for peri-implantitis and therefore the greater the difficulty to clean it?
- Could rough surfaces be a cause of peri-implant inflammation, since rougher surfaces are associated with a higher release of particles/ions?
The speaker explained that oxidised surfaces can improve protein adsorption and, consequently, cell adhesion (Hing et al., 2004, Anselme et al., 2000). At the same time, however, they may also facilitate bacterial colonisation (Derks et al., 2015).
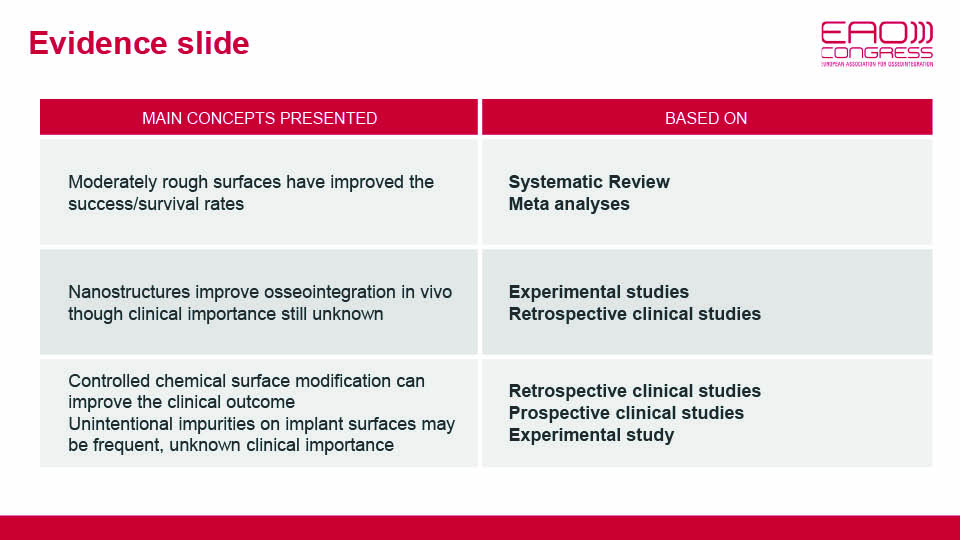
A recent meta-analysis compared turned and oxidised surfaces, and verified that turned implants have a significant risk ratio of 2.82, which puts them at a higher risk of failure than oxidised implants. However, the study found no difference in marginal bone levels between the two surfaces (Chrcanovic et al., 2016) (fig 1–2).
Another systematic review compared the long-term clinical results of different implant surfaces. Implants with anodised surfaces showed the fewest failures; whereas turned and blasted implants showed the lowest levels of marginal bone loss (Wennerberg et al., 2018). We can conclude that modern implants with a moderately rough surface have improved primary stability and can promote faster osseointegration, although mechanical and biological complications do still exist.
In 2007, the concept of nanoroughness (10–9 mm) was introduced to implant surface research (Webster et al., 2007). Nanoroughness was achieved by coating the implant surface (HA, TiO2). However, it was soon found that nanoroughness can spontaneously form over time, and was reported two weeks after surface contact with water or saline (Wennerberg et al., 2013) (fig 3–4).
An experimental study of rabbit tibias found a correlation between nanostructure surfaces and pull-out forces (Wennerberg et al., 2014). The potential benefits of this finding on the clinical environment has not yet been demonstrated.
Chemical modifications (fig 5–6)
Current research on implant surfaces primarily focuses on combining chemical and topographical modifications. One such example is the EISA technique (evaporation induced self-assembly), which aims to chemically coat implant surfaces with a thin, mesoporous TiO2 film which configures structures of 6nm. Apatite grows in the nano pores (Karlsson et al., 2012). These nano pores can be loaded with Mg ions, and this kind of modified surface was found to increase the strength of the bone-implant interface in an experimental study on rabbits (Galli et al. 2016).
Another research focus has been local drug delivery. In a recent study, a titanium mesoporous film covered with a polymer and with gold nano rods incorporated was found to be capable of delivering drugs to the surrounding area (Alenezi et al., 2019) (fig 7).
The speaker described another ongoing study in biodegradable implants which use Mg10Gd (magnesium gadolinium alloy). These implants could provide a useful alternative in large-scale bone reconstructions, where it would be advantageous to not have to remove them after bone healing.
Although polyetheretherketone (PEEK) alone doesn’t achieve osseointegration, it is susceptible to surface modifications. PEEK surfaces can be modified with hydroxyapatite (HA) nano coating to improve bone reactivity (Johansson et al., 2017). However, this method is still in an experimental stage, and PEEK needs to be researched and evaluated further before being introduced to clinical use (fig 8).
It is worth emphasising the recent investigation conducted by Duddeck and colleagues on the impurities and contamination of commercial implants. Implant surfaces from several well-known brands and copycat products from others were examined with SEM imaging and energy-dispersive X-ray spectroscopy. The study found many more impurities than expected. More contamination from both organic and inorganic sources was found in the copycat products compared with the originals, such as organic residue and unintended metal particles (e.g. iron or aluminium) (Duddeck et al., 2019). The true clinical significance of unclean implants remains unknown (fig 9–10).
Presentation figures
Fig 1
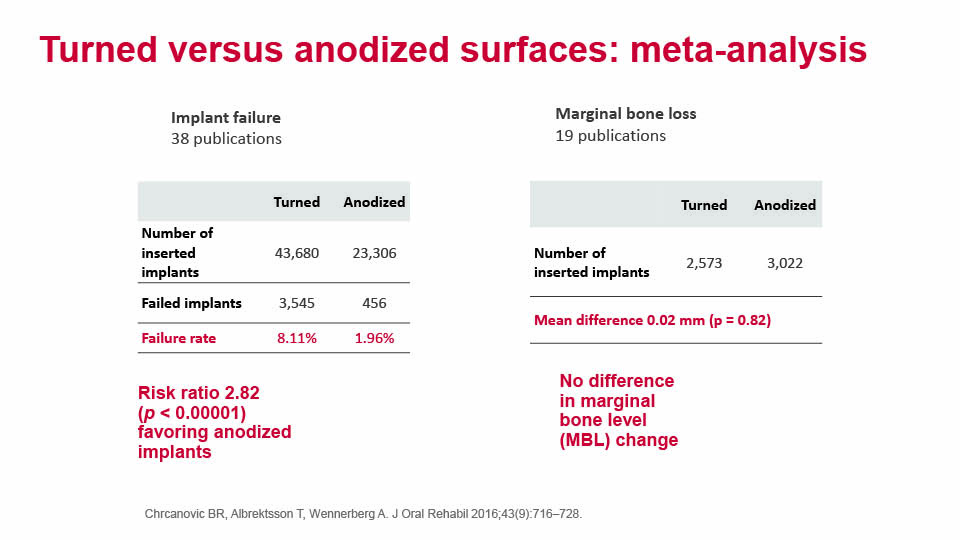
Fig 2

Fig 3

Fig 4

Fig 5
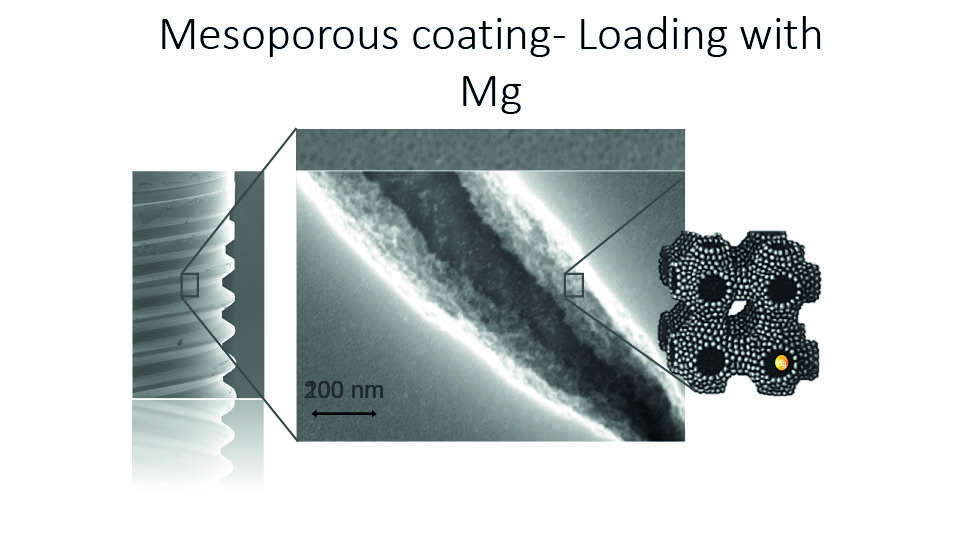
Fig 6
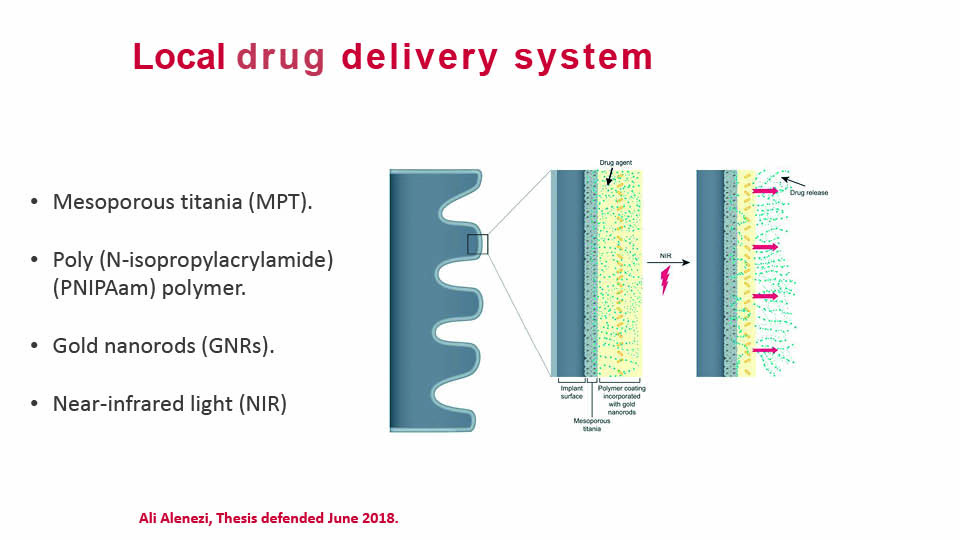
Fig 7

Fig 8
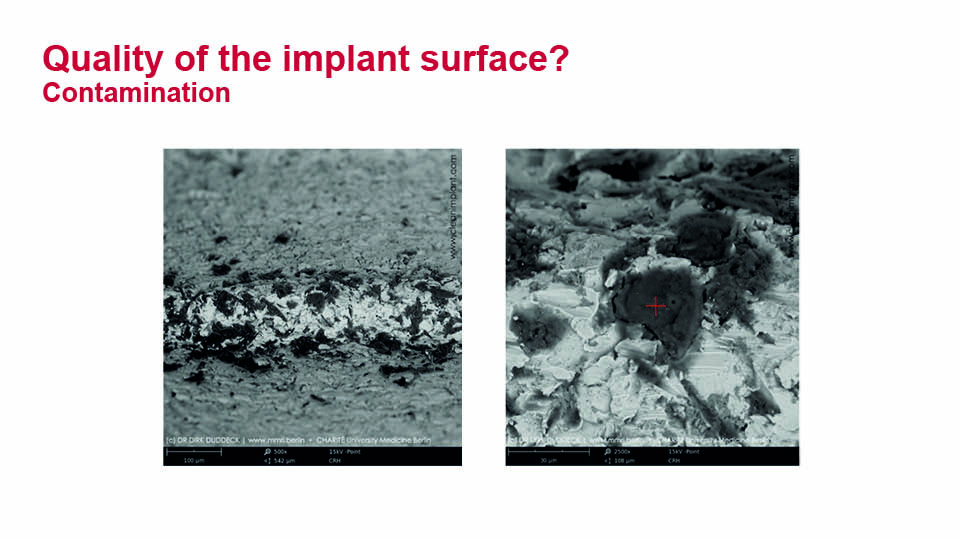
Fig 9

Fig 10
Evidence slide, Ann Wennerberg
References:
Anselme K. Osteoblast adhesion on biomaterials. Biomaterials 2000;21:667-81.
Alenezi A, Hulander M, Atefyekta S, Andersson M. Development of a photon induced drug-delivery implant coating. Mat Sci Eng C. 2019;98:619-27.
Chrcanovic BR, Albrektsson T, Wennerberg A. Turned versus anodized dental implants: a meta-analysis. J Oral Rehabil. 2016;43(9):716-28.
Derks J, Håkansson J, Wennström JL, Tomasi C, Larsson M, Berglundh T. Effectiveness of implant therapy analyzed in a Swedish population: early and late implant loss. J Dent Res. 2015;94 Suppl 3:44S-51S.
Duddeck DU, Albrektsson T, Wennerberg A, Larsson C, Beuer F. On the Cleanliness of Different Oral Implant Systems: A Pilot Study. J Clin Med. 2019;22;8(9):1280.
Galli S. On magnesium-containing implants for bone applications Thesis 2016. Malmö University. http://muep.mau.se/handle/2043/21277
Hing KA . Bone repair in the twenty-first century: biology, chemistry or engineering? Philos Trans A Math Phys Eng Sci. 2004;362(1825):2821-50.
Johansson, Pär. On hydroxyapatite modified peek implants for bone applications. Thesis 2017. Malmö University. http://muep.mau.se/handle/2043/23867
Karlsson J, Jimbo R, Fathali HM, Schwartz-Filho HO, Hayashi M, Halvarsson M, Wennerberg A, Andersson M. In vivo biomechanical stability of osseointegrating mesoporous TiO(2) implants. Acta Biomater. 2012;8(12):4438-46.
Webster TJ, Ahn ES. Nanostructured biomaterials for tissue engineering bone. Adv Biochem Eng Biotechnol. 2007;103:275-308.
Wennerberg A, Svanborg LM, Berner S, Andersson M. Spontaneously formed nanostructures on titanium surfaces. Clin Oral Implant Res. 2013;24(2):203-9.
Wennerberg A, Jimbo R, Stübinger S, Obrecht M, Dard M, Berner S. Nanostructures and hydrophilicity influence osseointegration: a biomechanical study in the rabbit tibia. Clin Oral Implants Res. 2014;25(9):1041-50.
Wennerberg A, Albrektsson T, Chrcanovic B. Long-term clinical outcome of implants with different surface modifications. Eur J Oral Implantol. 2018;11 Suppl 1:S123-S136.
Abutment surface modifications
The speaker focused on the biology behind the interaction between soft tissue and implant-supported prostheses (fig 11).
State of the art
According to the concept of ‘biological width’ (Hermann et al., 2001), it is well known that supracrestal connective tissue is crucial for maintaining the vertical dimension of soft tissue around abutments and vertical bone levels around implants. Experimental and clinical research has demonstrated that soft tissue morphogenesis around abutments occurs between 8 and 12 weeks (Berglundh et al., 2007; Tomasi et al., 2014). In the sulcus, connective tissue is surrounded by epithelium that adheres to the abutment surface with a thin layer of proteoglycans (Iglhaut et al., 2014).
Some articles describe the pre-operative clinical characteristics of the soft tissue (Kan et al., 2010, Linkevicius et al., 2015). These articles describe thick and thin biotypes, but do not show any correlation between biotype and individual healing patterns. Regarding abutment material, a recent systematic review found no difference between titanium and zirconium abutments (Sanz-Sánchez et al., 2018).
Soft tissue integration has many variables: the host and their phenotype; the characteristics of the prosthetic material (aka ‘the guest’); the workflow we adopt; and the microbiome (‘the antagonist’).
The host
There is no difference in the number of cells in thick and thin biotypes. Thick biotypes seem to have more extracellular matrix and a thicker lamina, which are involved in the formation of hyaluronic acid and other proteins potentially related to adhesion processes. Both phenotypes are determined by gene expression, and modulated by epigenetics via the synthesis of methyl and acetyl groups. These groups either deactivate or boost protein production respectively.
Some researchers have found that peroxisome proliferator-activated (PPA) receptors may be involved in soft tissue healing, which may explain why some cases heal correctly and others do not (Aghaei et al., 2016; Korbecki et al., 2019). When PPA receptor methylation was observed and highly expressed, even in thin biotypes, improved healing and increased connective tissue height was more likely. On the other hand, when there was no PPA receptor methylation or expression, a longer junctional epithelium and bone resorption was reported (fig 12–13).
The guest
The macro, micro and nano characteristics of abutment materials are important. Perhaps equally vital, however, is the timing of treatment. From the literature, it appears that clinicians have a strong influence over soft tissue healing during the prosthetic workflow stages (Tallarico et al., 2018).
Timing
In fact, repeated disconnection of abutments can disrupt tissue healing, and lead to a longer junctional epithelium (instead of connective tissue) being formed around the abutment. To prevent this from happening, the speaker proposed different strategies to maximise connective tissue adhesion to the abutment:
- Using an intra-surgical impression and ensuring the definitive abutment is ready at the time of re-opening
- An intermediate abutment can be used to transform bone-level implants in tissue-level implants
- A digital impression can also be taken at the time of implant placement, allowing the screw-retained crown to be prepared in advance of the second surgery
Morphology
The macro morphology of the abutment also appears to influence soft tissue characteristics. A recent study comparing narrow and wide abutments found no differences regarding aesthetics or periodontal parameters, although narrow abutments showed less marginal bone loss (Canullo et al., 2019a). The speaker explained that the reason for this may be that connective fibres have more space and can better rearrange around the abutment, forming a stronger tissue seal.
Surface
Improved connective tissue healing was observed during early stages (3–6 months) in abutments with modified surfaces (Pesce et al., 2019). This is probably because tissue can be stabilised on the surface in a more coronal position, thus taking advantage of any grooves or surface irregularities (Piattelli et al., 2011). In the long term, however, rough surfaces did not seem to provide clinical advantages in soft tissue (unless used in cases involving ‘one abutment one time’ after bioactivation) (Canullo et al., 2019b).
Surface energy decreases over time, caused by atmospheric pollution and can even lead to new abutments becoming hydrophobic (Sawase et al., 2000; Canullo et al., 2013). Bioactivation of the abutment surface can improve cell adhesion quantitatively, in that a greater number of cells will be involved, and qualitatively, as a better arrangement of this adhesion can be achieved (Garcia 2017; Canullo et al., 2019c). Finally, bioactivation can be applied to a three-dimensional geometry of surfaces, leading to faster cell differentiation, clinically resulting in a larger band of connective tissue (Canullo et al., 2019d).
The antagonist
Bacteria compete with fibroblasts for space on the abutment surface (Neoh et al., 2012). When the abutment surface is decontaminated, fibroblasts can attach (even in cases involving rough surfaces) (Massucci et al., 2019). The speaker outlined a strict decontamination protocol which is recommended when placing the abutment and placing the prothesis.
Fig 14
Presentation figures
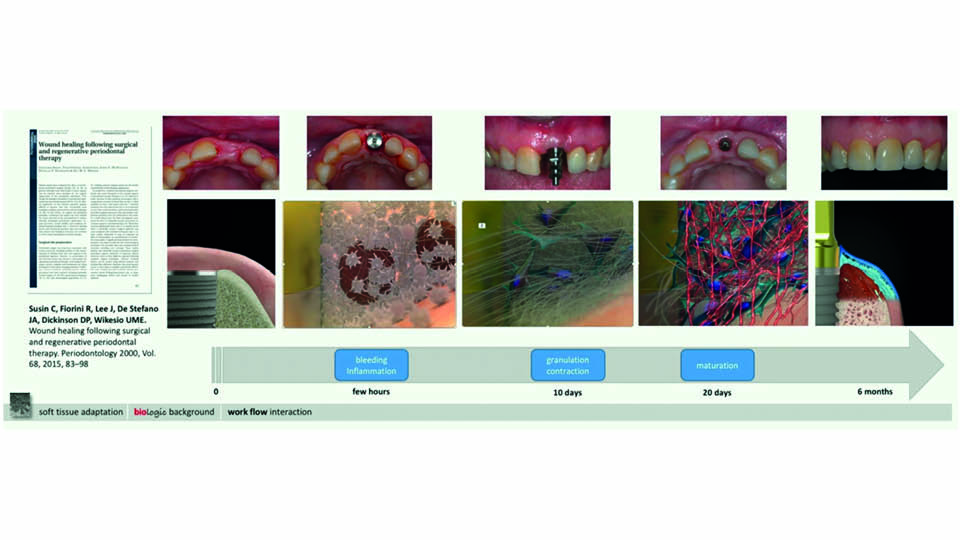
Fig 11: Peri-implant soft tissue healing
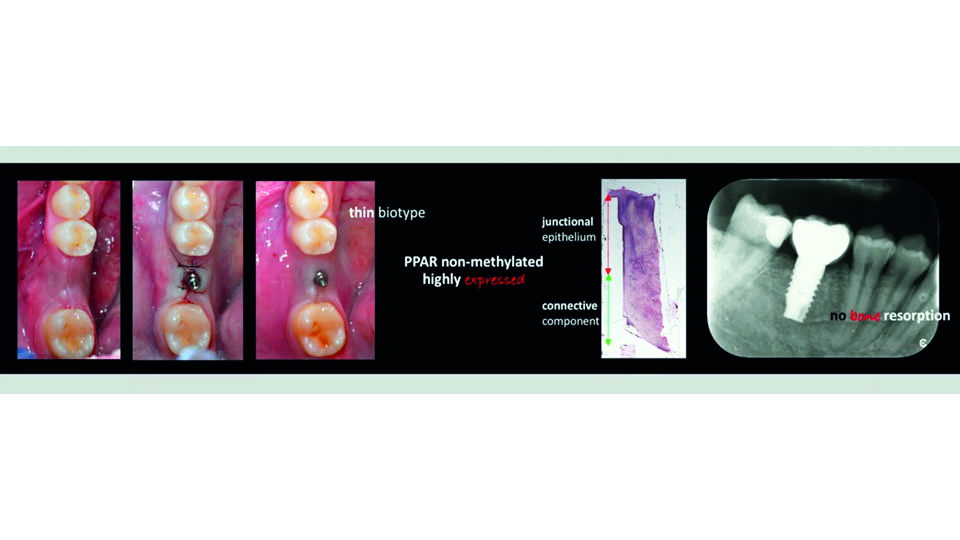
Fig 12: PPARs high: improved healing
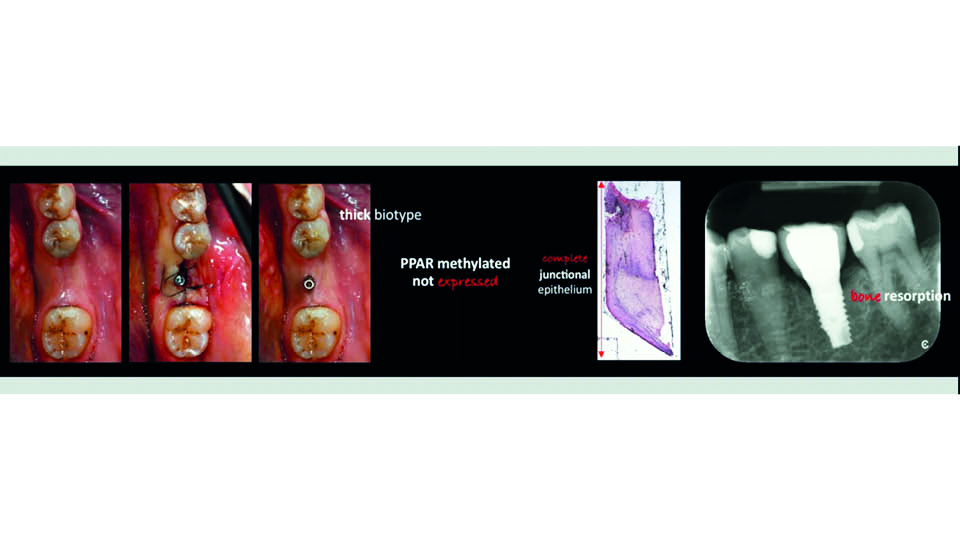
Fig 13: PPARs low: defective healing

Fig 14, evidence of the concepts presented
References:
Aghaei S, Nilforoushzadeh MA, Aghaei M. The role of peroxisome proliferator-activated receptor-coactivator-1 gene in skin aging. J Res Med Sci. 2016;14;21:36.
Berglundh T, Abrahamsson I, Welander M, Lang NP, Lindhe J. Morphogenesis of the peri-implant mucosa: an experimental study in dogs. Clin Oral Implant Res. 2007;18(1):1-8.
Canullo L, Micarelli C, Iannello G. Microscopical and chemical surface characterization of the gingival portion and connection of an internal hexagon abutment before and after different technical stages of preparation. Clin Oral Implant Res. 2013;24(6):606-11.
Canullo L, Penarrocha-Oltra D, Marchionni S, Bagán L, Peñarrocha-Diago MA, Micarelli C. Soft tissue cell adhesion to titanium abutments after different cleaning procedures: preliminary results of a randomized clinical trial. Med Oral Patol Oral Cir Bucal. 2014 Mar 1;19(2):e177-83.
Canullo, L, Pesce P, Patini R, Antonacci, Tommasato G. What are the effects of different abutment morphology on peri-implant hard and soft tissues behavior? A systematic review and meta-analysis. Int J Prosthod 2019 A (in review).
Canullo L, Manning M, Santori G, Rakic M, Sculean A, Pesce P. Titanium abutment surfaces modifications and peri-implant tissue behaviour. A systematic review and meta-analysis. Clin Oral Inv. 2019 B (in review).
Canullo L, Genova T, Gross E, Pradies G, Muzzi M, Mussano F. Fibroblast interaction with different abutment surfaces: in vitro study. Clin Impl Dent Rel Res 2019 C (in review)
Canullo L, Zarauz C, Pesce R, Tomasi C, Lattanzio R, Penarrocha-Oltra D, Lezzi G. Soft tissue healing around abutment surfaces: histological outcomes of a RCT. Clin Impl Dent Rel Res 2019 D (in review)
Garcia B, Camacho F, Peñarrocha D, Tallarico M, Perez S, Canullo L. Influence of plasma cleaning procedure on the interaction between soft tissue and abutments: a randomized controlled histologic study. Clin Oral Implant Res. 2017;28(10):1269-77.
Hermann JS, Buser D, Schenk RK, Schoolfield JD, Cochran DL. Biologic Width around one- and two-piece titanium implants. Clin Oral Implant Res. 2001;12(6):559-71.
Iglhaut G, Schwarz F, Winter RR, Mihatovic I, Stimmelmayr M, Schliephake H. Epithelial attachment and downgrowth on dental implant abutments–a comprehensive review. J Esthet Restor Dent. 2014;26(5):324-31.
Kan JY, Morimoto T, Rungcharassaeng K, Roe P, Smith DH. Gingival biotype assessment in the esthetic zone: visual versus direct measurement. Int J Periodontics Restorative Dent. 2010;30(3):237-43.
Korbecki J, Bobinski R, Dutka M. Self-regulation of the inflammatory response by peroxisome proliferator-activated receptors. Inflamm Res. 2019;68(6):443-58.
Linkevicius T, Puisys A, Steigmann M, Vindasiute E, Linkeviciene L. Influence of Vertical Soft Tissue Thickness on Crestal Bone Changes Around Implants with Platform Switching: A Comparative Clinical Study. Clin Implant Dent Relat Res. 2015;17(6):1228-36.
Massucci L, Patini R, Quarant G, Troiano G, Pesce P, Ravidá A, Penarrocha-Oltra D, Canullo L. Culturomic and qPCR analyses for early contamination of abutments with different surfaces: a RCT. Int J Oral Impl 2019 (in review).
Neoh KG, Hu X, Zheng D, Kang ET. Balancing osteoblast functions and bacterial adhesion on functionalized titanium surfaces. Biomaterials. 2012;33(10):2813-22.
Pesce P, Menini M, Tommasato G, Patini R, Canullo L. Influence of modified titanium abutment surface on peri-implant soft tissue behaviour: A systematic review of histological findings. Int J Oral Implantol (New Malden). 2019;12(4):419-29.
Piattelli A, Pontes AE, Degidi M, Iezzi G. Histologic studies on osseointegration: soft tissues response to implant surfaces and components. A review. Dent Mater. 2011;27(1):53-60.
Sanz-Sánchez I, Sanz-Martín I, Carrillo de Albornoz A, Figuero E, Sanz M. Biological effect of the abutment material on the stability of peri-implant marginal bone levels: A systematic review and meta-analysis. Clin Oral Implant Res. 2018;29 Suppl 18:124-44.
Sawase T, Wennerberg A, Hallgren C, Albrektsson T, Baba K. Chemical and topographical surface analysis of five different implant abutments. Clin Oral Implant Res. 2000;11(1):44-50.
Tallarico M, Caneva M, Meloni SM, Xhanari E, Covani U, Canullo L. Definitive Abutments Placed at Implant Insertion and Never Removed: Is It an Effective Approach? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Oral Maxillofac Surg. 2018;76(2):316-324.
Tomasi C, Tessarolo F, Caola I, Wennström J, Nollo G, Berglundh T. Morphogenesis of peri-implant mucosa revisited: an experimental study in humans. Clin Oral Implant Res. 2014;25(9):997-1003.
How implant and abutment design influence clinical outcomes
Clinical outcomes can be evaluated by a variety of diverse parameters. Does implant or abutment design matter in relation to the desired outcome? And what are the roles of biology, surgery and prosthodontics on clinical outcomes?
Abutment length
A retrospective study found that shorter abutments were associated with increased bone loss (Vervaeke et al., 2014). Short abutments are typically used when implants are positioned too high for the bone level and/or when the covering mucosa is thin. In these cases, bone remodelling can result in thread exposure. In a prospective randomized split mouth study it was confirmed that placing implants deeper, to allow 4 mm of soft tissue attachment, yielded an absence of thread exposure and less marginal bone loss (Vervaeke S, Matthys C, Nassar R, Christiaens V, Cosyn J, De Bruyn H. J Clin Periodontol. 2018 May;45(5):605-612. doi: 10.1111/jcpe.12871. Epub 2018 Feb 23). However, thread exposure has not yet been clearly associated with clinical peri-implantitis given the short term follow-up.
Preserving marginal bone
Implant macro design may also play an important role in bone loss. When the thread pitch is too high, bone loss increases and implant survival rates decrease; 0.6mm is considered the optimum inter-thread distance (Vandeweghe et al., 2012a).
Both machined and rough implant surfaces showed the same outcomes in immediate and conventional loading, regardless of design. Microthread necks did not make a difference to marginal bone level preservation (Van de Velde et al., 2010; Ravald et al., 2013).
Primary stability
Several changes have been made to macro designs in an attempt to improve primary stability. The surgical protocol should be adapted to each patient and their individual bone morphology. To this end, drills and steps for each procedure should be patient-guided instead of company-guided. The speaker cautioned that the learning curve when moving to another implant design should also be taken into account. Primary stability is a well-recognised benefit for immediate loading; but in delayed loading protocols, macro design modifications are not as important as we might think.
Implant-abutment interface
A recent study showed that implant-abutment interface does not influence pink aesthetic scores (PES) compared with other implant designs. The study did find that the interface appears to have a negative effect on marginal bone loss in flat-to-flat and platform switching designs compared with conical designs (Cooper et al., 2019). However, it should be noted that some features of the study may not have been well controlled, and so we cannot definitively conclude that conical designs are associated with less bone than other kinds of platforms.
Platform switching
It has been demonstrated that platform switched implants can better maintain marginal bone levels (Vandeweghe et al., 2012b). When used with correct biological implant placement approaches, according to soft tissue thickness, platform-switching may play an important role in compromised patients (smokers, periodontally involved patients, diabetics, etc) (fig 15–16).
Angular correction
Some implants have angle correction designs which allow us to position implants so that they are surrounded by bone, without an unfavourable prosthetic axis and allowing us to avoid fenestration. These implants are especially recommended in the anterior maxilla (Vandeweghe et al., 2012c; Vandeweghe et al., 2013). Results have shown that buccal bone performations requiring bone regenerative techniques are avoided when this angular connection within the implant is used, because the implant threads are completely covered by bone. However, crestal bone thickness was not as wide as simply placing the implant in a more palatal position. Angle-corrected implants have been also been the subject of a study investigating all-on-four implants (Van Weehaeghe et al., 2017).
Reverse tapered body
The speaker described another new implant design which has a wider apical half and thinner coronal half. This allows about 50% greater primary stability to be achieved in extraction sockets than conventional tapered implants with the same dimension, thus facilitating immediate loading. In combination with the angular connection within the implant, this furthermore improves the aesthetic outcome (fig 17–18).
Wider body implants
In the posterior area, immediate implant placement techniques may be improved by using an implant with a wider diameter than is conventional. With this design, implant geometry is optimised to engage the perimeter of the molar socket and fill it. In this way, remarkable primary stability can be achieved, allowing immediate loading in most cases (Hattingh et al., 2018) (fig 19).
Mini implants
Thin implants are being investigated in ‘fragile’ patients who are considered unsuitable for conventional implant designs. They can be placed in a minimally invasive way and, although the failure rate was around 16%, they have been shown to improve the quality of life of these patients with compromised bone volume (fig 20).
A recent clinical study made a quadruple comparison: implants with and without microthreads, and implants with external and internal connections. The same solid conical abutment was fitted in all groups at the moment of implant placement. After one year no differences were reported in bone remodelling, regardless of implant design (Glibert et al., 2018). Bone response does not seem to be influenced by these design factors; correct biological positioning of implants seems to be the main prognostic factor for preserving marginal bone.
Abutment design
Several studies have been conducted on abutment design and have come to the same conclusion: convex/concave and regular/curved abutments all show similar marginal bone loss levels and mucosal margin positions (Patil et al., 2014; Koutouzis et al., 2019). Additionally, abutment modifications do not affect the patient’s satisfaction with the aesthetic outcome (Patil et al., 2016, Patil et al., 2017).
The angle of the abutment emergence profile may influence marginal bone loss, but not the level of mucosa. Abutments with an angled emergence profile of more than 30º can encourage marginal bone loss and may also be considered a risk indicator for peri-implantitis. Further, convex profiles can pose an additional risk for bone‐level implants, but not for tissue‐level implants (Katafuchi et al., 2018) (fig 21).
These are the conclusions of the presentation:
- biology determines long-term clinical outcomes
- commercially driven dogmas are not scientifically proven
- there is not such a thing as ‘one implant for all indications’ – an individual approach is best for implant selection
- angled implants combine the best surgical option with the best prosthetic option
- site-specific implants are growing in popularity, as they can enlarge indications and enhance outcomes in compromised situations
- large emergence profiles and cemented restorations have a higher risk for peri-implant issues, so it is generally better to screw!
- it is not the engineering algorithms, but the clinical algorithms which will prevail
Presentation figures
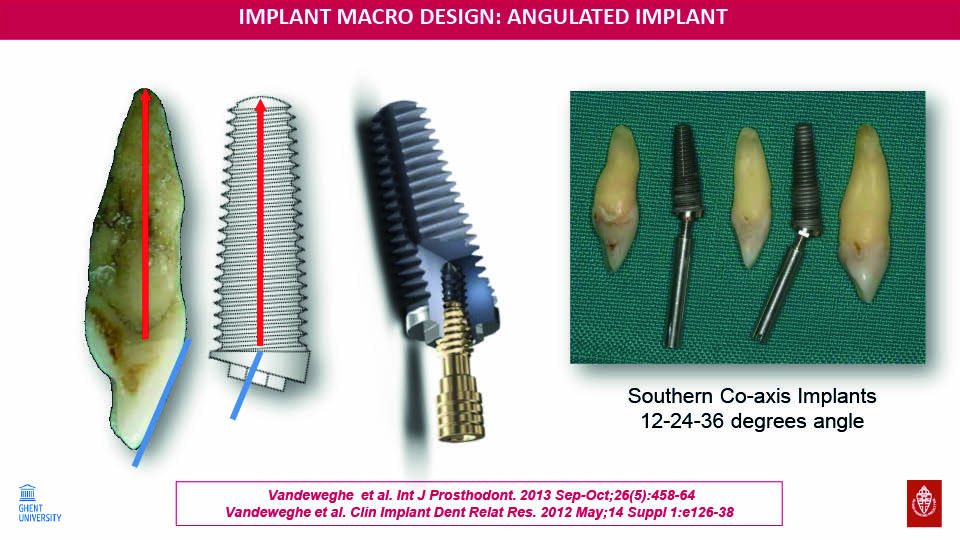
Fig 15
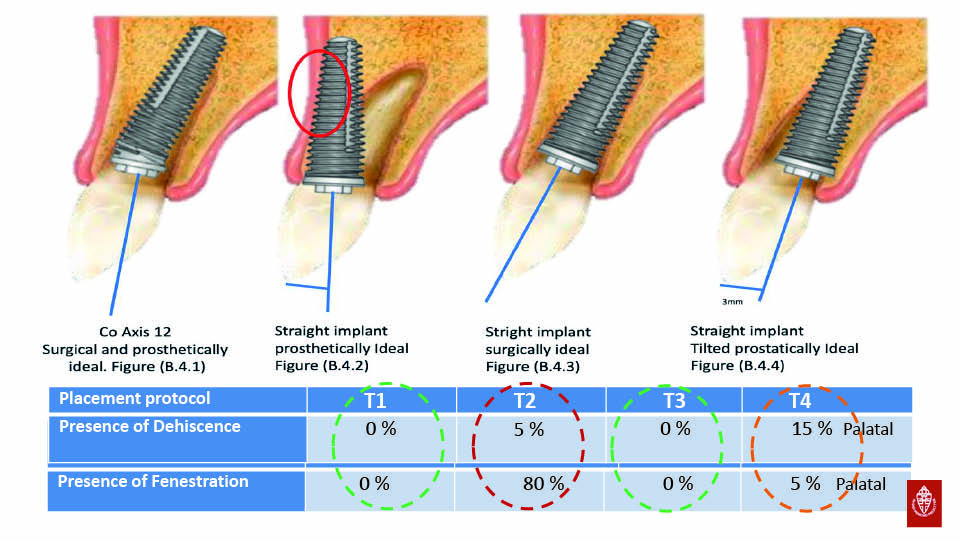
Fig 16: Angular correction incorporated
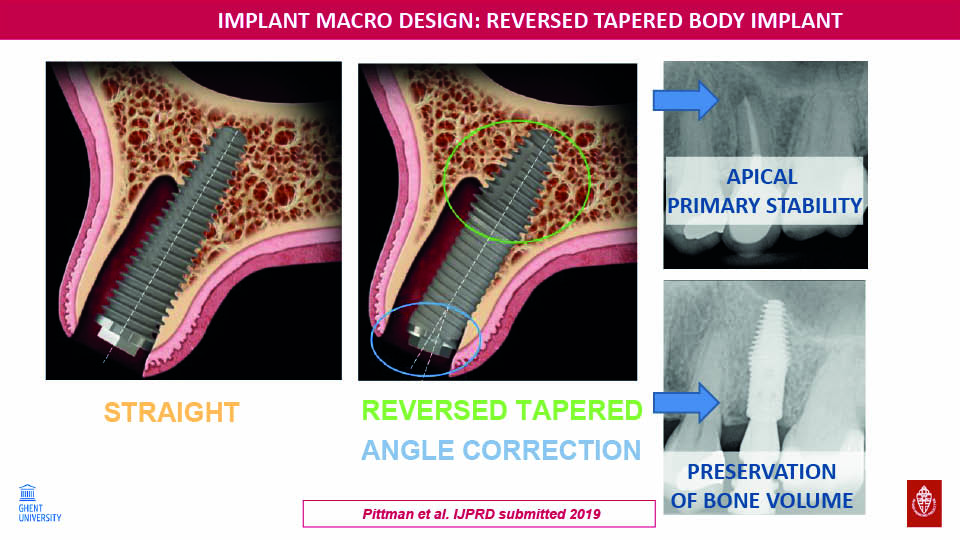
Fig 17
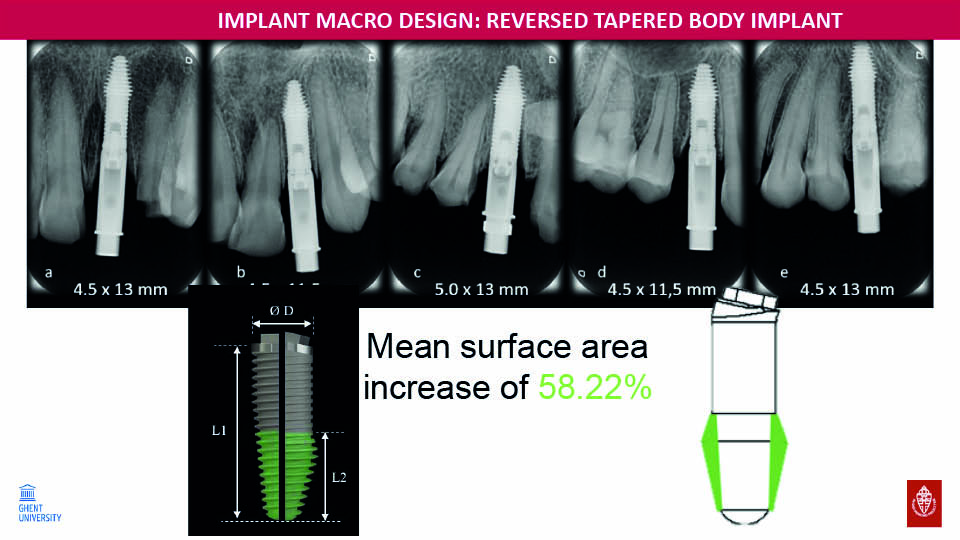
Fig 18
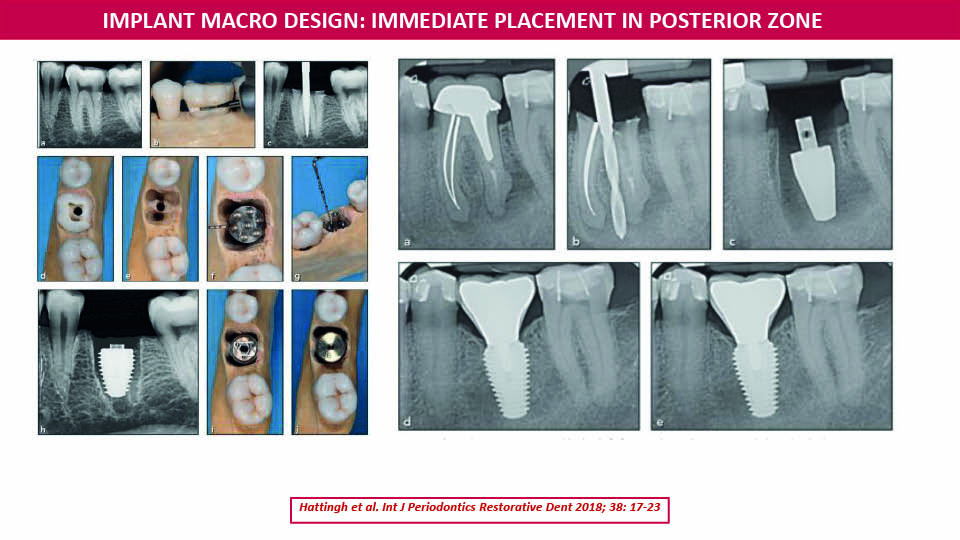
Fig 19
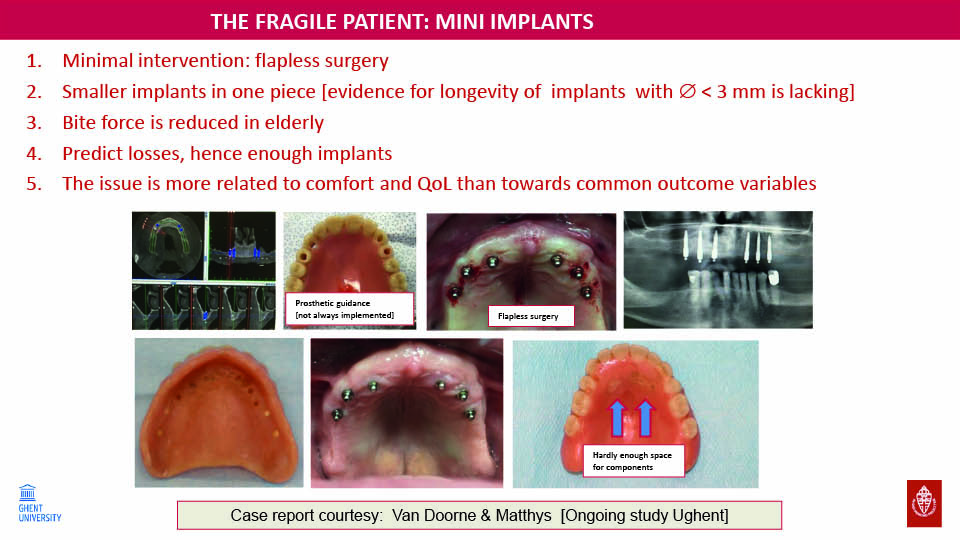
Fig 20

Fig 21: Issues covered in the presentation
References:
Cooper LF, Reside G, Stanford C, Barwacz C, Feine J, Nader SA, Scheyer T, McGuire M. Three-Year Prospective Randomized Comparative Assessment of Anterior Maxillary Single Implants with Different Abutment Interfaces. Int J Oral Maxillofac Implants. 2019;34(1):150-8.
Glibert M, Vervaeke S, Jacquet W, Vermeersch K, Östman PO, De Bruyn H. A randomized controlled clinical trial to assess crestal bone remodeling of four different implant designs. Clin Implant Dent Relat Res. 2018;20(4):455-62.
Hattingh AC, De Bruyn H, Ackermann A, Vandeweghe S. Immediate Placement of Ultrawide-Diameter Implants in Molar Sockets: Description of a Recommended Technique. Int J Periodontics Restorative Dent. 2018;38(1):17-23.
Katafuchi M, Weinstein BF, Leroux BG, Chen YW, Daubert DM. Restoration contour is a risk indicator for peri-implantitis: A cross-sectional radiographic analysis. J Clin Periodontol. 2018;45(2):225-32.
Koutouzis T, Adeinat B, Ali A. The influence of abutment macro-design on clinical and radiographic peri-implant tissue changes for guided, placed, and restored implants: A 1-year randomized controlled trial. Clin Oral Implant Res. 2019;30(9):882-91.
Patil RC, den Hartog L, van Heereveld C, Jagdale A, Dilbaghi A, Cune MS. Comparison of two different abutment designs on marginal bone loss and soft tissue development. Int J Oral Maxillofac Implants. 2014;29(3):675-81.
Patil R, den Hartog L, Dilbaghi A, de Jong B, Kerdijk W, Cune MS. Papillary fill response in single-tooth implants using abutments of different geometry. Clin Oral Implant Res. 2016;27(12):1506-10.
Patil R, Gresnigt MMM, Mahesh K, Dilbaghi A, Cune MS. Esthetic Evaluation of Anterior Single-Tooth Implants with Different Abutment Designs-Patients’ Satisfaction Compared to Dentists’ Observations. J Prosthodont. 2017;26(5):395-8.
Ravald N, Dahlgren S, Teiwik A, Gröndahl K. Long-term evaluation of Astra Tech and Brånemark implants in patients treated with full-arch bridges. Results after 12-15 years. Clin Oral Implants Res. 2013;24(10):1144-51.
Van de Velde T, Collaert B, Sennerby L, De Bruyn H. Effect of implant design on preservation of marginal bone in the mandible. Clin Implant Dent Relat Res. 2010;12(2):134-41.
Van Weehaeghe M, De Bruyn H, Vandeweghe S. A prospective, split-mouth study comparing tilted implants with angulated connection versus conventional implants with angulated abutment. Clin Implant Dent Relat Res. 2017;19(6):989-96.
Vandeweghe S Cosyn J, Thevissen E, Teerlinck J, De Bruyn H. The influence of implant design on bone remodeling around surface-modified Southern Implants®. Clin Implant Dent Relat Res. 2012A;14(5):655-62.
Vandeweghe S, De Bruyn H. A within-implant comparison to evaluate the concept of platform switching: a randomised controlled trial. Eur J Oral Implantol. 2012B;5(3):253-62.
Vandeweghe S, Cosyn J, Thevissen E, Van den Berghe L, De Bruyn H. A 1-year prospective study on Co-Axis implants immediately loaded with a full ceramic crown. Clin Implant Dent Relat Res. 2012C;14 Suppl 1:e126-38.
Vandeweghe S, Nicolopoulos C, Thevissen E, Jimbo R, Wennerberg A, De Bruyn H. Immediate loading of screw-retained all-ceramic crowns in immediate versus delayed single implant placement. Int J Prosthodont. 2013;26(5):458-64.
Vervaeke S, Dierens M, Besseler J, De Bruyn H. The influence of initial soft tissue thickness on peri-implant bone remodeling. Clin Implant Dent Relat Res. 2014;16(2):238-47.



