Dealing with complications
How to treat peri-implantitis
Current approaches for treating peri-implantitis (PI)
The speaker asked ‘What should we bear in mind when we are faced with treating peri-implantitis?’ The four main factors he highlighted were (fig 1):
- plaque control capacity, both by the patient and hygienist or dentist
- expected reduction of probing depths
- possibility of implant surface decontamination
- feasibility of bone reconstruction
We also need to achieve the disease resolution. Currently, healing is considered to be a combined clinical outcome based on (Sanz & Chapple, 2012) (fig 2):
- absence of bleeding/suppuration
- probing depth not more than 5mm
- no additional bone loss
Several years ago, defects were classified as class I (vertical) or class II (horizontal) (Schwarz et al., 2007). In reality, however, many defects are a combination of the two: 60% of infrabony defects are associated with dehiscence; and 80% of supracrestal ones are combined with vertical defects. Despite this, the classification remains useful for guiding and informing the recommended surgical procedure.
Is non-surgical treatment worth it?
Non-surgical treatment shows little predictability and new technologies have not been proved to be more beneficial than conventional debridement (Faggion et al., 2014). Non-surgical protocols have a wide variable range for achieving disease resolution: 38 to 49% cases. In spite of the apparent inefficacy of these treatments, however, they should always be performed as a first step to prepare the surgical procedure.
Is there a decision rule for deciding to explant?
Once non-surgical treatment options have been exhausted, the decision needs to be made whether to explant or to treat. The speaker described the decision rule for explantation or surgical treatment thus: if bone loss accounts for more than two thirds of the implant surface or ≧ 7mm, explantation is recommended. If either of these measures are lower, then surgery should be considered (Schwartz & Sanz-Sánchez, 2015).
What is the best surgical approach?
Potential surgical procedures could be to use an access flap, or to adopt a resective (including osseous reshaping) or reconstructive approach. The decision of which to use depends on the morphology of the defect. In pure horizontal class II defects, an access flap or resective approach would be the best option. In a pure intrabony class I defect, a reconstructive approach. In combined cases, a combined approach is recommended (fig 3–4).
An access flap approach consists of eliminating granulation tissue, decontaminating the implant surface and suturing the flap in its initial position. It is useful in aesthetic areas when reconstruction is not feasible and/or bone is not going to be reshaped. With similar protocols, the 5-year success rate[1] of this approach reached 63% (Heitz-Mayfield et al., 2018) or, in a different study, 30% (Berglundh et al., 2018). In the latter study better results were obtained when implants had a machined surface.
Resective surgery modifies the contours of hard or soft tissues, aiming to reduce probing depths and improve cleansability. The reported outcomes of this approach highlight the fact that when pocket depth is reduced together with professional supportive therapy, good results can be maintained in the long-term (Serino et al., 2015).
Is there any other way to improve my results? (fig 5)
Implantoplasty has been suggested to improve treatment predictability. Indeed, smoothing the surface implant can improve results (Romeo et al., 2005) and can achieve a success rate over 80% (Bianchini et al., 2019). For this procedure, technical limitations should be taken into account when the prosthesis cannot be removed. There are also drawbacks to note: there is a 32% decrease in the fracture resistance of internal connection implants, especially in narrow diameter implants (Costa et al., 2018); and the leakage of titanium particles that can then get stuck in surrounding tissues, whose long-term biological effects are still unknown (Stavropoulos et al., 2019). Implantoplasty can also be performed in conjunction with reconstruction in combined defects, on exposed surfaces, the supracrestal component, and buccal or lingual dehiscence with 79% success (Schwarz et al., 2017) (fig 6).
Another attempt to improve the clinical results of access surgery has involved antimicrobials. There have been three recent RCTs on the subject. The systemic administration of amoxicillin seems to have improved results with rough surface implants. When azithromyzin was administered systemically, the reported differences were not significant. Only the local application of minocycline gel yielded better results than controls (Cha et al., 2019).
Although the literature on this approach is scarce, modifying soft tissue quantity and/or quality has been claimed to improve results of peri-implantitis treatment. A recent systematic review revealed that when an autogenous soft tissue graft was added to the apically positioned flap, the bone level and peri-implant gingival index were both significantly better (Thoma et al., 2018). Some ongoing RCTs seem to be pointing to similar results. This systematic review deals with prevention, not with treatment of peri-implantitis.
[1] Three success parameters were used: no BoP/SoP; PD ≧ 5mm ; no further Rx bone loss
Presentation figures
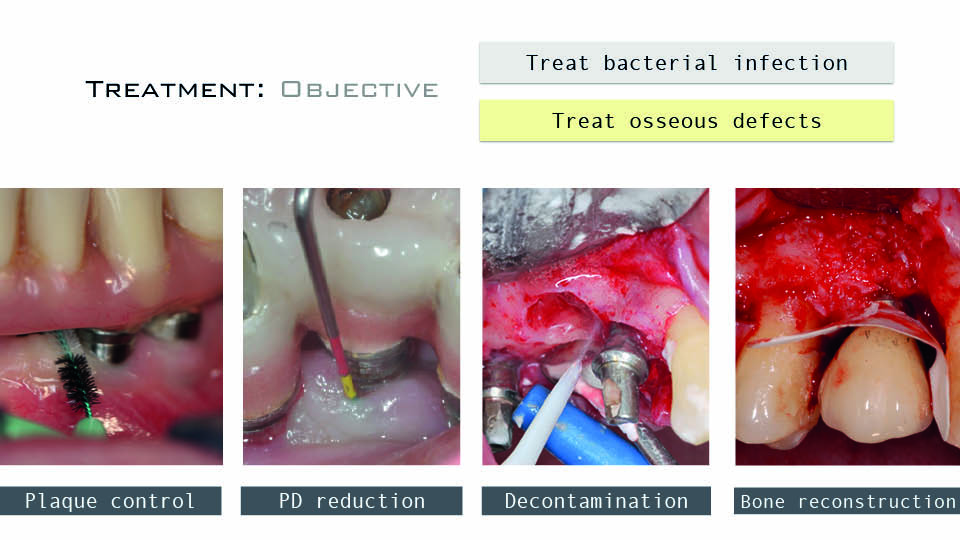
Fig 1: The four factors in treatment of peri-implantitis

Fig 2: Healing is a combined clinical outcome
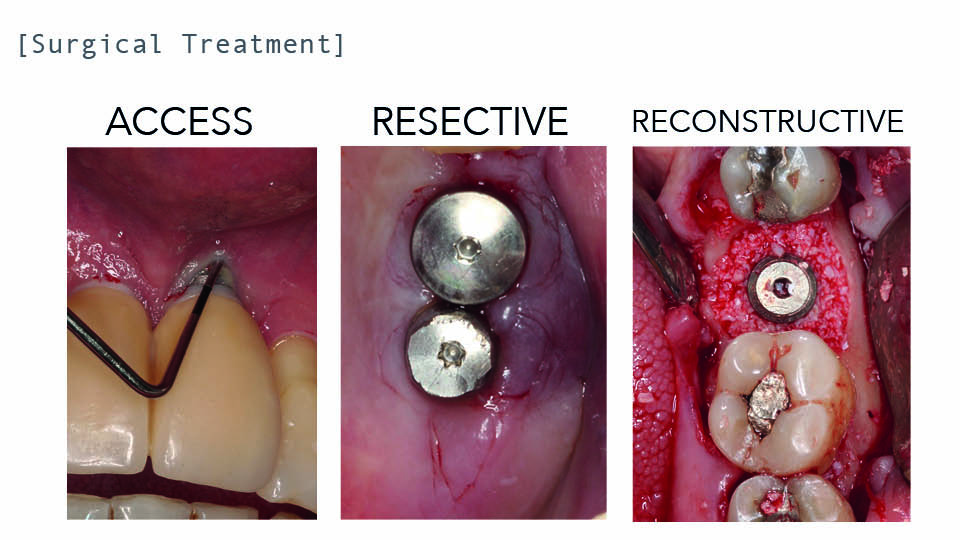
Fig 3: Different surgical approaches
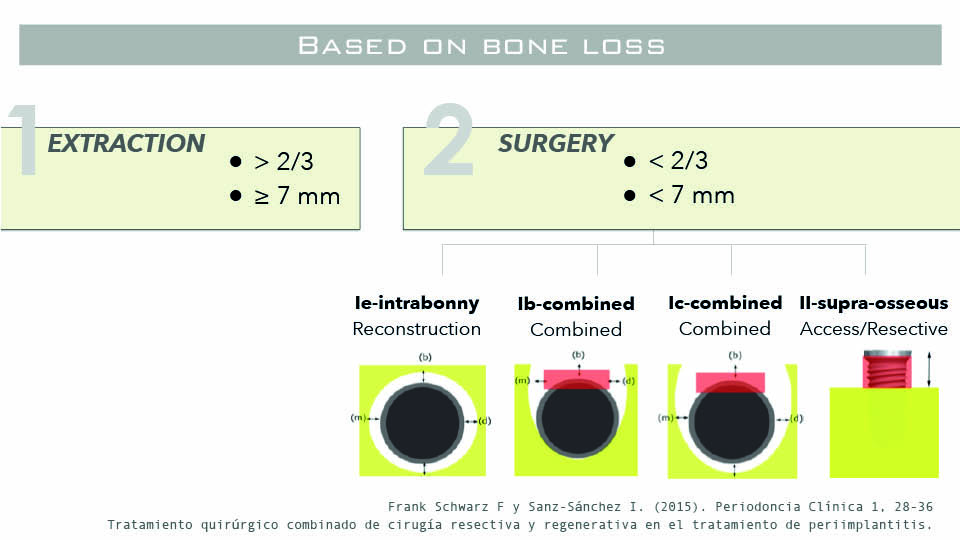
Fig 4: Surgical approach according to the defect morphologies
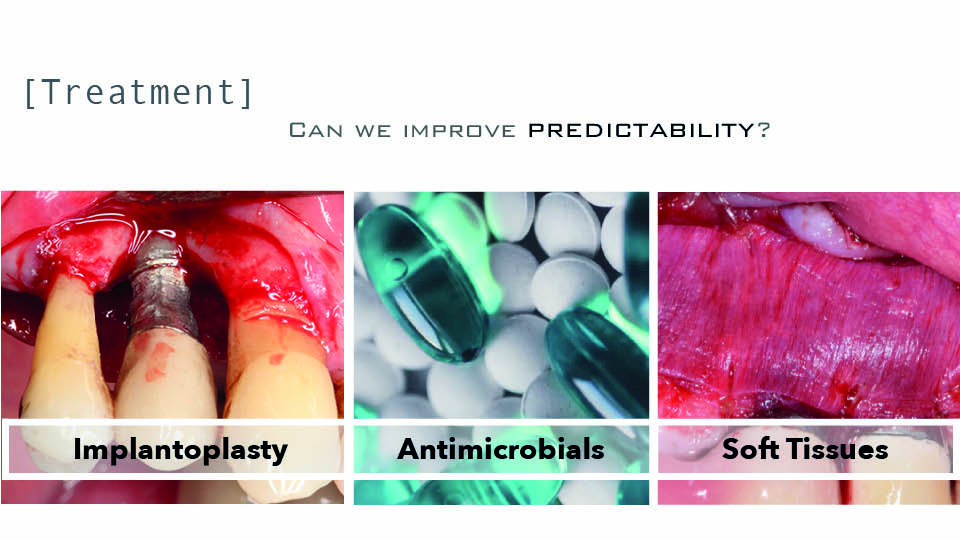
Fig 5: Adjunctive treatment measures
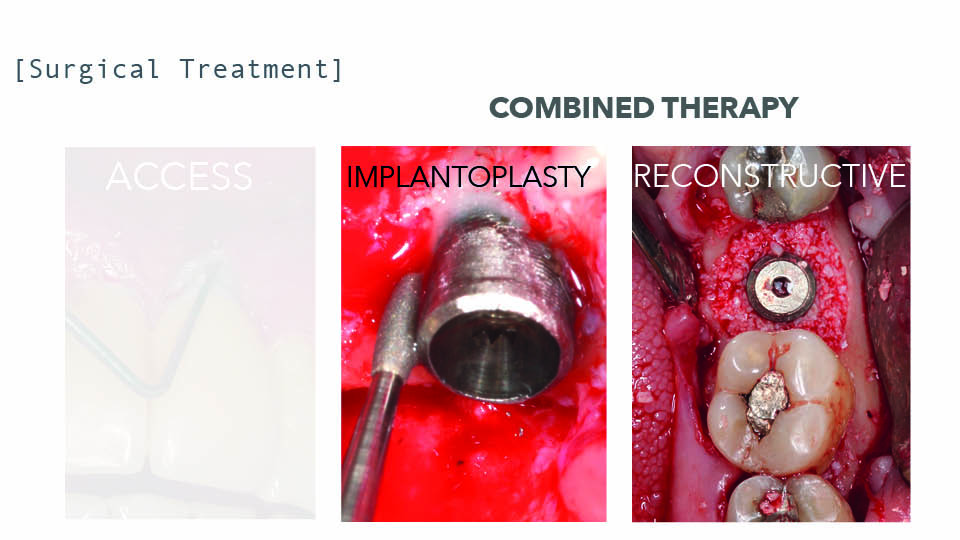
Fig 6: Implantoplasty vs reconstructive therapy
References:
Berglundh T, Wennstron JL, Lindhe J. Long-term outcome of surgical treatment of periimplantitis: A 2-11-year retrospective study. Clin Oral Implant Res 2018;29(4):404-10.
Bianchini MA, Galarraga-Vinueza ME, Apaza-Bedoya K, De Souza JM, Magini R, Schwarz F. Two to six-year disease resolution and marginal bone stability rates of a modified resective implantoplasty therapy in 32 peri-implantitis cases. Clin Implant Dent Relat Res, 2019;21(4):758-65
Cha JK, Lee JS, Kim CS. Surgical Therapy of Peri-Implantitis with Local Minocycline: A 6-Month Randomized Controlled Clinical Trial. J Dent Res, 2019;98(3):288-95.
Costa-Berenguer X, García-García M, Sanchez-Torres A, Sanz-Alonso M, Figueiredo R, Valmaseda-Castellón E. Effect of implantoplasty on fracture resistance and surface roughness of standard diameter dental implants. Clin Oral Implant Res 2018;29(1):46-54.
Faggion CM Jr, Listl S, Frühauf N, Chang HJ, Tu YK. A systematic review and Bayesian network meta-analysis of randomized clinical trials on non-surgical treatments for peri-implantitis. J Clin Periodontol, 2014;41(10):1015-25.
Heitz-Mayfield LJ, Salvi, GE Mombelli A et al. Supportive peri-implant therapy following anti-infective surgical peri-implantitis treatment: 5-year survival and success. Clin Oral Implant Res 2018;29(1);1-6.
Romeo E, Ghisolfi M, Murgolo N, Chiaoasco M, Lops D, Vogel G. Therapy of peri‐implantitis with resective surgery: A 3‐year clinical trial on rough screw‐shaped oral implants. Part I: clinical outcome. Clin Oral Implants Res, 2005;16(1):9-18.
Sanz M, Chapple IL; Working Group 4 of the VIII European Workshop on Periodontology. J Clin Periodontol, 2012;39 Suppl 12:202-6.
Schwarz F, Herten M, Sager M, Bieling K, Sculean A, Becker J. Comparison of naturally occurring and ligature‐induced peri‐implantitis bone defects in humans and dogs. Clin Oral Implants Res, 2007;18:161–170.
Schwarz F & Sanz-Sánchez I. Tratamiento quirúrgico combinado de cirugía respectiva y regenerativa en el tratamiento de periimplantitis. Perio Clin, 2015;1(1):28-36
Schwarz F, John G, Schmucker A, Sahm m, Becker J. Combined surgical therapy of advanced periimplantitis evaluating two methods of surface decontamination: a 7-year follow-up observation. J Clin Periodontol, 2017;44(3):337-342
Serino G, Turri A, Lang NP. Maintenance therapy in patients following the surgical treatment of periimplantitis: a 5-year follow-up study. Clin Oral Implant Res, 2015;26(8):950-6.
Stavropoulos A, Bertl K, Eren S, Gotfredsen K. Mechanical and biological complications after implantoplasty: A systematic review. Clin Oral Implant Res, 2019;30(9):833-48.
Thoma DS, Naenny N, Figuero E, Hammerle CH, Schwarz F, Jung RE, Sanz-Sánchez I. Effects of soft tissues augmentation procedures on peri-implant health or disease: a systematic review and meta-analysis. Clin Oral Implant Res, 2018;29 Suppl 15:32-49.
Regenerative approaches for peri-implantitis
Regeneration in periodontics refers to obtaining new periodontal attachment. With regard to peri-implantitis (PI), regeneration means re-osseointegration (i.e. new direct contact of bone tissue to a previously contaminated titanium surface). As osseointegration is assessed histologically, it cannot be part of clinical evaluations and goals. Rather than talking about ‘regenerative’ approaches we should instead talk about ‘reconstructive’ approaches.
When can we use a reconstructive instead of a resective approach?
There are many cases (especially in the aesthetic area) where we would like to prevent soft tissue recession. In a corresponding scenario in the periodontal patient, a reconstructive approach would be selected; with PI it is similar. We want to minimise soft tissue recession, which is what the patient is looking for, and this means a happier patient in the end.
The speaker explained that healing is strongly influenced by implant surface characteristics, while the superiority of any grafting material remains to be demonstrated.
In a recent experimental study on the surgical therapy of peri-implantitis (Almohandes et al., 2019), significantly better healing was observed at implants with a relatively smooth surface compared to those with a moderately rough surface. The type of bone substitute used was not associated with additional benefits; the use (or not) of a membrane also made no difference. The pronounced influence of surface roughness may have been due to the greater ability for the smooth surface to be cleaned and decontaminated.
Is there evidence supporting the use of grafting materials vs open flap debridement in PI treatment?
A recent systematic review and meta-analysis could only include three controlled studies (Tomasi et al., 2019). No differences were found with regard to improvement of inflammatory parameters, but the scarcity of data made it hard to draw conclusions, especially when only radiographic levels were measured; and no study addressed either recession or patient-reported outcome measures (PROMs). Hence, evidence is currently lacking on improved aesthetic outcomes or PROMs following reconstructive therapy compared with open flap debridement. It is clear that recession should be assessed as an additional outcome measure in future studies. Soft tissue levels should be evaluated as outcomes after PI treatment.
Is there a superior decontamination method to use?
Although evidence is still limited, the speaker stated that the decontamination protocol used in an ongoing multicentre study comparing the addition or not of a xenograft (ClinicalTrial.gov, NCT03077061) appears superior to other alternatives. The protocol does not include implantoplasty, but uses titanium brushes in an effort to decontaminate but not destroy the implant surface, as a recent in vitro study has shown (Cha et al., 2019). This suggested protocol has been shown to be effective as concluded by an RCT (de Tapia et al., 2019).
References:
Almohandes A, Carcuac O, Abrahamsson I, Lund H, Berglundh T. Re‐osseointegration following reconstructive surgical therapy of experimental peri‐implantitis. A pre‐clinical in vivo study. Clin Oral Implant Res. 2019;30:447–56.
Cha JK, Paeng K, Jung UW, Choi SH, Sanz M, Sanz‐Martín I. The effect of five mechanical instrumentation protocols on implant surface topography and roughness: A scanning electron microscope and confocal laser scanning microscope analysis. Clin Oral Implant Res, 2019;30:578–87.
de Tapia B, Valles C, Ribeiro-Amaral T, Mor C, Herrera D, Sanz M, Nart J. The adjunctive effect of a titanium brush in implant surface decontamination at peri‐implantitis surgical regenerative interventions: A randomized controlled clinical trial. J Clin Periodontol, 2019;46:586–96.
Tomasi C, Regidor E, Ortiz-Vigón A, Derks J. Efficacy of reconstructive surgical therapy at peri-implantitis-related bone defects. A systematic review and meta-analysis. J Clin Periodontol, 2019;46 Suppl 21:340-56.
ClinicalTrial.gov identifier NCT03077061. “Reconstructive surgical therapy of peri-implantitis-related osseous defects: A multicenter randomized controlled clinical trial”. Sponsored by Göteborg University.



